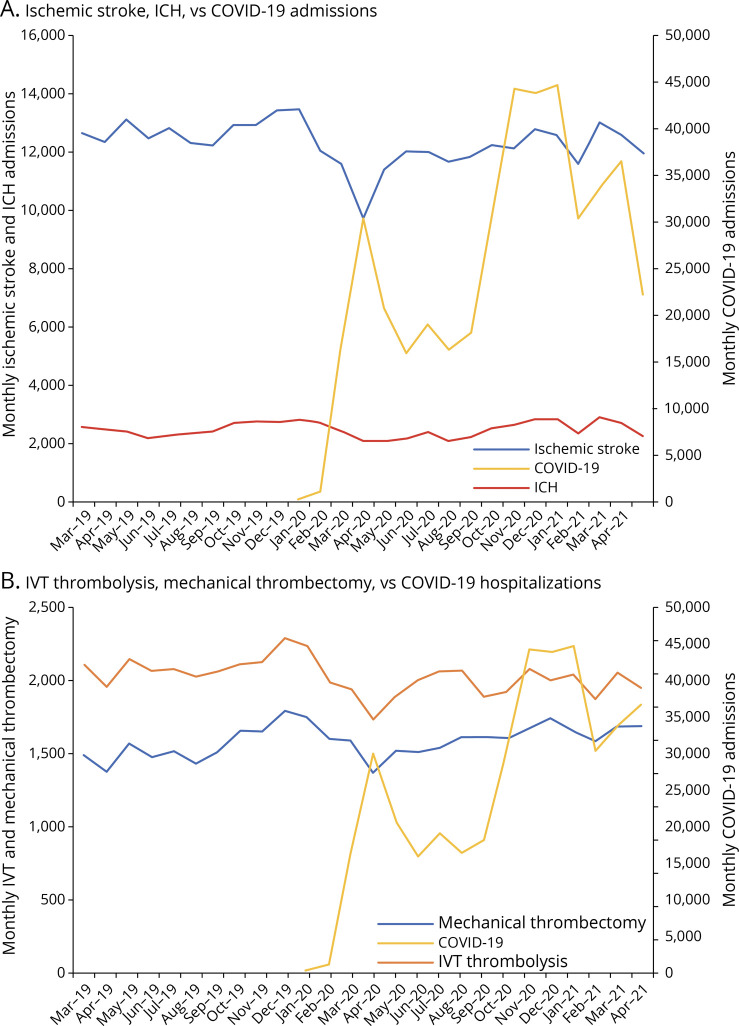
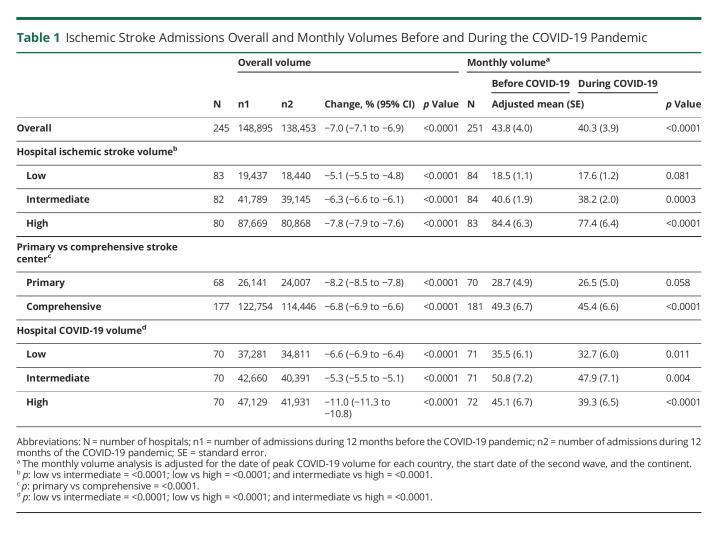
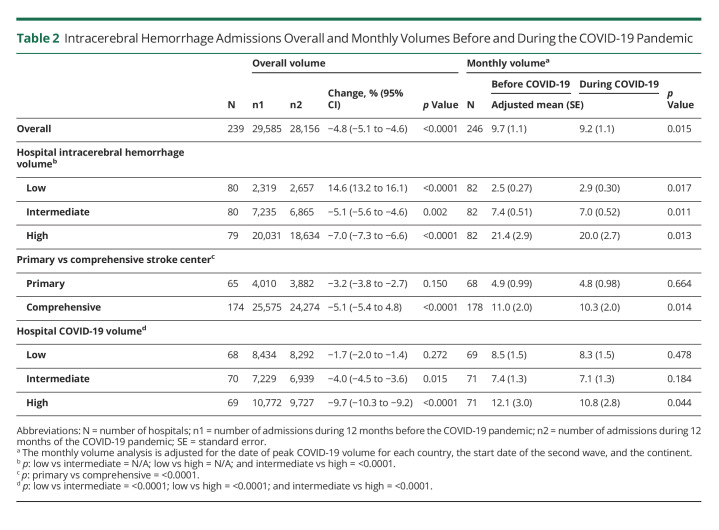
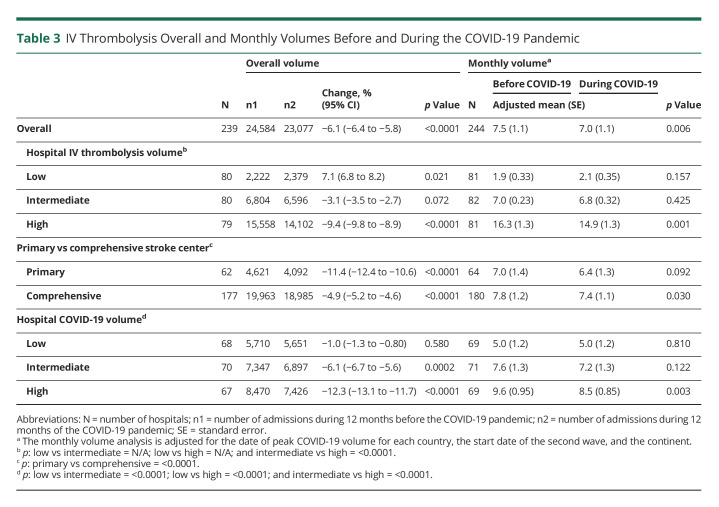
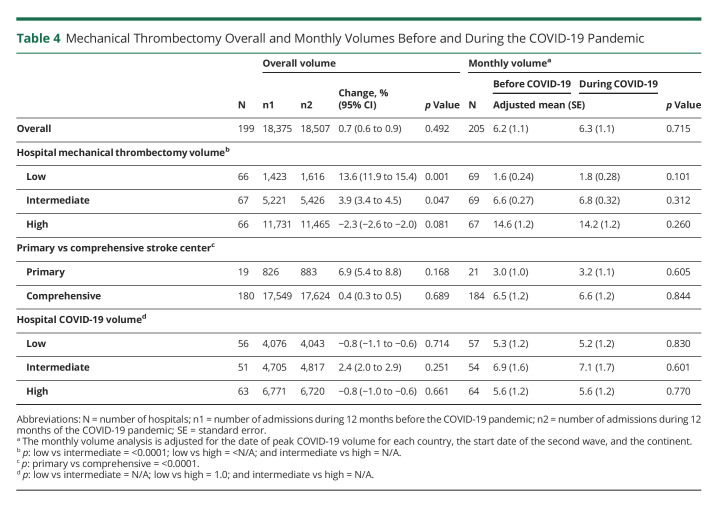
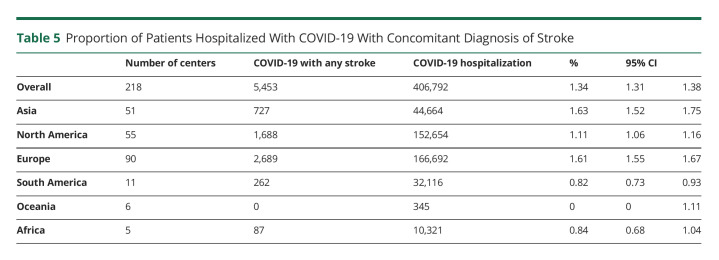
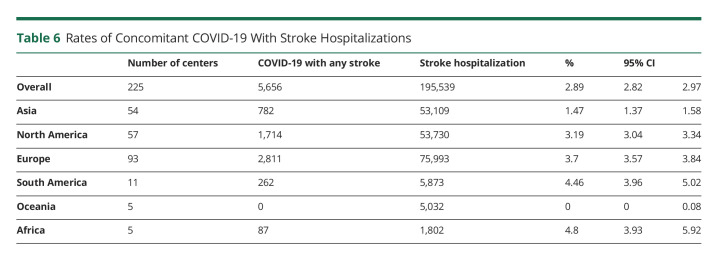
Muhammad M Qureshi
Muhammad M Qureshi, MBBS, MPH
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Piers Klein
Piers Klein, MA
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hiroshi Yamagami
Hiroshi Yamagami, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Robert Mikulik
Robert Mikulik, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anna Czlonkowska
Anna Czlonkowska, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mohamad Abdalkader
Mohamad Abdalkader, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Petra Sedova
Petra Sedova, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anvitha Sathya
Anvitha Sathya
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hannah C Lo
Hannah C Lo, BS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ossama Yassin Mansour
Ossama Yassin Mansour, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Husitha Reddy Vanguru
Husitha Reddy Vanguru, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Emilie Lesaine
Emilie Lesaine, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Georgios Tsivgoulis
Georgios Tsivgoulis, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aaron I Loochtan
Aaron I Loochtan, DO
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jelle Demeestere
Jelle Demeestere, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ken Uchino
Ken Uchino, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Violiza Inoa
Violiza Inoa, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nitin Goyal
Nitin Goyal, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Andreas Charidimou
Andreas Charidimou, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
James E Siegler
James E Siegler
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shadi Yaghi
Shadi Yaghi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Diana Aguiar de Sousa
Diana Aguiar de Sousa, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mahmoud H Mohammaden
Mahmoud H Mohammaden, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Diogo C Haussen
Diogo C Haussen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Espen Saxhaug Kristoffersen
Espen Saxhaug Kristoffersen, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Virginia Pujol Lereis
Virginia Pujol Lereis, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sergio Daniel Scollo
Sergio Daniel Scollo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Bruce C V Campbell
Bruce C V Campbell, MBBS, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Alice Ma
Alice Ma, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
James Orton Thomas
James Orton Thomas, BMed
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mark W Parsons
Mark W Parsons, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shaloo Singhal
Shaloo Singhal, MBBS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lee-Anne Slater
Lee-Anne Slater, MBBS, FRANZCR, Mmed
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rodrigo Tomazini Martins
Rodrigo Tomazini Martins, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Chris Enzinger
Chris Enzinger, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Thomas Gattringer
Thomas Gattringer, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aminur Rahman
Aminur Rahman, MD, FCPS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Thomas Bonnet
Thomas Bonnet, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Noemie Ligot
Noemie Ligot, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sylvie De Raedt
Sylvie De Raedt, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Robin Lemmens
Robin Lemmens, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Peter Vanacker
Peter Vanacker, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Fenne Vandervorst
Fenne Vandervorst, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adriana Bastos Conforto
Adriana Bastos Conforto, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Raquel CT Hidalgo
Raquel CT Hidalgo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Luciana de Oliveira Neves
Luciana de Oliveira Neves, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rodrigo Targa Martins
Rodrigo Targa Martins, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Daissy Liliana Mora Cuervo
Daissy Liliana Mora Cuervo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Leticia C Rebello
Leticia C Rebello, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Igor Bessa Santiago
Igor Bessa Santiago, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Isabelle Lameirinhas da Silva
Isabelle Lameirinhas da Silva, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Teodora Sakelarova
Teodora Sakelarova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rosen Kalpachki
Rosen Kalpachki, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Filip Alexiev
Filip Alexiev, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Luciana Catanese
Luciana Catanese, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Elena Adela Cora
Elena Adela Cora, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mayank Goyal
Mayank Goyal, MD, PhD, FRCPC
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael D Hill
Michael D Hill, MD, MSc, FRCPC
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael E Kelly
Michael E Kelly, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Houman Khosravani
Houman Khosravani, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Pascale Lavoie
Pascale Lavoie, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lissa Peeling
Lissa Peeling, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aleksandra Pikula
Aleksandra Pikula, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rodrigo Rivera
Rodrigo Rivera, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hui-Sheng Chen
Hui-Sheng Chen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yimin Chen
Yimin Chen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Xiaochuan Huo
Xiaochuan Huo, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Zhongrong Miao
Zhongrong Miao, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shuiquan Yang
Shuiquan Yang, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marina Roje Bedekovic
Marina Roje Bedekovic, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marina Bralic
Marina Bralic, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hrvoje Budincevic
Hrvoje Budincevic, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Angel Basilio Corredor-Quintero
Angel Basilio Corredor-Quintero, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Osvaldo E Lara-Sarabia
Osvaldo E Lara-Sarabia, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Martin Cabal
Martin Cabal, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Dusan Tenora
Dusan Tenora, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Petr Fibrich
Petr Fibrich, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Roman Herzig
Roman Herzig, MD, PhD, FESO, FEAN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Helena Hlaváčová
Helena Hlaváčová, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Emanuela Hrabanovska
Emanuela Hrabanovska, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
David Hlinovsky
David Hlinovsky, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lubomir Jurak
Lubomir Jurak, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jana Kadlcikova
Jana Kadlcikova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Igor Karpowicz
Igor Karpowicz, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lukas Klecka
Lukas Klecka, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Martin Kovar
Martin Kovar, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
David Lauer
David Lauer, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jiri Neumann
Jiri Neumann, MD, FESO
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hana Palouskova
Hana Palouskova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Martin Reiser
Martin Reiser, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Petra Rekova
Petra Rekova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Vladimir Rohan
Vladimir Rohan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ondrej Skoda
Ondrej Skoda, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Miroslav Škorňa
Miroslav Škorňa, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lenka Sobotková
Lenka Sobotková, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Martin Sramek
Martin Sramek, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lenka Zakova
Lenka Zakova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hanne Christensen
Hanne Christensen, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nicolas Drenck
Nicolas Drenck, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Helle Klingenberg Iversen
Helle Klingenberg Iversen, MD, DMSci
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Thomas Clement Truelsen
Thomas Clement Truelsen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Troels Wienecke
Troels Wienecke, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Khalid Sobh
Khalid Sobh, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Pauli Ylikotila
Pauli Ylikotila, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kemal Alpay
Kemal Alpay, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Daniel Strbian
Daniel Strbian, MD, PhD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Patricia Bernady
Patricia Bernady, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Philippe Casenave
Philippe Casenave, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Maria Dan
Maria Dan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jean-Marc Faucheux
Jean-Marc Faucheux, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jean-Christophe Gentric
Jean-Christophe Gentric, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Elsa Magro
Elsa Magro, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Candice Sabben
Candice Sabben, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Peggy Reiner
Peggy Reiner, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Francois Rouanet
Francois Rouanet, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ferdinand O Bohmann
Ferdinand O Bohmann, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Stefan Boskamp
Stefan Boskamp, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Joshua Mbroh
Joshua Mbroh, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Simon Nagel
Simon Nagel, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Christian H Nolte
Christian H Nolte
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Peter A Ringleb
Peter A Ringleb, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael Rosenkranz
Michael Rosenkranz, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sven Poli
Sven Poli, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Götz Thomalla
Götz Thomalla, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Theodoros Karapanayiotides
Theodoros Karapanayiotides, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ioanna Koutroulou
Ioanna Koutroulou, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Odysseas Kargiotis
Odysseas Kargiotis, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lina Palaiodimou
Lina Palaiodimou, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jose Dominguo Barrientos Guerra
Jose Dominguo Barrientos Guerra, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Vikram Huded
Vikram Huded, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Bindu Menon
Bindu Menon, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shashank Nagendra
Shashank Nagendra, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Chintan Prajapati
Chintan Prajapati, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
PN Sylaja
PN Sylaja, MBBS, MD, DM
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nyoman Angga Krishna Pramana
Nyoman Angga Krishna Pramana, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Achmad Firdaus Sani
Achmad Firdaus Sani, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Abdoreza Ghoreishi
Abdoreza Ghoreishi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mehdi Farhoudi
Mehdi Farhoudi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Elyar Sadeghi Hokmabadi
Elyar Sadeghi Hokmabadi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Tariq Abu Raya
Tariq Abu Raya, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shani Avnery Kalmanovich
Shani Avnery Kalmanovich, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Levite Ronen
Levite Ronen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sergiu Ionut Sabetay
Sergiu Ionut Sabetay, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Maurizio Acampa
Maurizio Acampa, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Alessandro Adami
Alessandro Adami, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lucio Castellan
Lucio Castellan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marco Longoni
Marco Longoni, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Raffaele Ornello
Raffaele Ornello, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Leonardo Renieri
Leonardo Renieri, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Claudia Rolla Bigliani
Claudia Rolla Bigliani, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michele Romoli
Michele Romoli, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Simona Sacco
Simona Sacco, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Andrea Salmaggi
Andrea Salmaggi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Davide Sangalli
Davide Sangalli, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Andrea Zini
Andrea Zini, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ryosuke Doijiri
Ryosuke Doijiri, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hiroki Fukuda
Hiroki Fukuda, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Toshiyuki Fujinaka
Toshiyuki Fujinaka, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kyohei Fujita
Kyohei Fujita, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hirotoshi Imamura
Hirotoshi Imamura, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nobuyuki Sakai
Nobuyuki Sakai, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Takuya Kanamaru
Takuya Kanamaru, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Naoto Kimura
Naoto Kimura, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ryuhei Kono
Ryuhei Kono, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kosuke Miyake
Kosuke Miyake, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Manabu Sakaguchi
Manabu Sakaguchi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kenichiro Sakai
Kenichiro Sakai, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kazutaka Sonoda
Kazutaka Sonoda, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kenichi Todo
Kenichi Todo, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Fumio Miyashita
Fumio Miyashita, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Naoki Tokuda
Naoki Tokuda, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yuji Matsumaru
Yuji Matsumaru, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shoji Matsumoto
Shoji Matsumoto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nobuyuki Ohara
Nobuyuki Ohara, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Seigo Shindo
Seigo Shindo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yohei Takenobu
Yohei Takenobu, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Takeshi Yoshimoto
Takeshi Yoshimoto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kazunori Toyoda
Kazunori Toyoda, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Takeshi Uwatoko
Takeshi Uwatoko, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yoshiki Yagita
Yoshiki Yagita, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Takehiro Yamada
Takehiro Yamada, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nobuaki Yamamoto
Nobuaki Yamamoto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ryoo Yamamoto
Ryoo Yamamoto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yukako Yazawa
Yukako Yazawa, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yuri Sugiura
Yuri Sugiura, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Peter Kuria Waweru
Peter Kuria Waweru, MBChB, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jang-Hyun Baek
Jang-Hyun Baek, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Si Baek Lee
Si Baek Lee, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kwon-Duk Seo
Kwon-Duk Seo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sung-Il Sohn
Sung-Il Sohn, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anita Ante Arsovska
Anita Ante Arsovska, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yong Chieh Chan
Yong Chieh Chan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Wan Asyraf Wan Zaidi
Wan Asyraf Wan Zaidi, Mmed
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ainul Syahrilfazli Jaafar
Ainul Syahrilfazli Jaafar, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Fernando Gongora-Rivera
Fernando Gongora-Rivera, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Manuel Martinez-Marino
Manuel Martinez-Marino, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adrian Infante-Valenzuela
Adrian Infante-Valenzuela, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Stanislav Groppa
Stanislav Groppa, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Pavel Leahu
Pavel Leahu, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jonathan M Coutinho
Jonathan M Coutinho, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Leon A Rinkel
Leon A Rinkel, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Diederik WJ Dippel
Diederik WJ Dippel, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Dianne HK van Dam-Nolen
Dianne HK van Dam-Nolen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Annemarei Ranta
Annemarei Ranta, MD, PhD, FRACP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Teddy Y Wu
Teddy Y Wu, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Tajudeen Temitayo Adebayo
Tajudeen Temitayo Adebayo
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Abiodun H Bello
Abiodun H Bello
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ernest Okwundu Nwazor
Ernest Okwundu Nwazor, MBBS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Taofiki Ajao Sunmonu
Taofiki Ajao Sunmonu, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kolawole Wasiu Wahab
Kolawole Wasiu Wahab, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ole Morten Ronning
Ole Morten Ronning, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Else Charlotte Sandset
Else Charlotte Sandset, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Amal M Al Hashmi
Amal M Al Hashmi, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Saima Ahmad
Saima Ahmad, MBBS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Umair Rashid
Umair Rashid, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Liliana Rodriguez-Kadota
Liliana Rodriguez-Kadota, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Miguel Ángel Vences
Miguel Ángel Vences, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Patrick Matic Yalung
Patrick Matic Yalung, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jon Stewart Hao Dy
Jon Stewart Hao Dy, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Maria Carissa Pineda-Franks
Maria Carissa Pineda-Franks, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Christian Oliver Co
Christian Oliver Co, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Waldemar Brola
Waldemar Brola, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aleksander Debiec
Aleksander Debiec, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Malgorzata Dorobek
Malgorzata Dorobek, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michal Adam Karlinski
Michal Adam Karlinski, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Beata M Labuz-Roszak
Beata M Labuz-Roszak, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anetta Lasek-Bal
Anetta Lasek-Bal, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Halina Sienkiewicz-Jarosz
Halina Sienkiewicz-Jarosz, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jacek Staszewski
Jacek Staszewski, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Piotr Sobolewski
Piotr Sobolewski, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marcin Wiacek
Marcin Wiacek, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Justyna Zielinska-Turek
Justyna Zielinska-Turek, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Andre Pinho Araujo
Andre Pinho Araujo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mariana Rocha
Mariana Rocha, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Pedro Castro
Pedro Castro, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Vitor Tedim Cruz
Vitor Tedim Cruz, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Paulo Venancio Ferreira
Paulo Venancio Ferreira, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Patricia Ferreira
Patricia Ferreira, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ana Paiva Nunes
Ana Paiva Nunes, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Luisa Fonseca
Luisa Fonseca, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
João Pedro Marto
João Pedro Marto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Teresa Pinho e Melo
Teresa Pinho e Melo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Miguel Rodrigues
Miguel Rodrigues, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
M Luis Silva
M Luis Silva, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adela Dimitriade
Adela Dimitriade, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Cristian Falup-Pecurariu
Cristian Falup-Pecurariu, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
May Adel Hamid
May Adel Hamid, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Narayanaswamy Venketasubramanian
Narayanaswamy Venketasubramanian, FRCP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Georgi Krastev
Georgi Krastev, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Miroslav Mako
Miroslav Mako, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Oscar Ayo-Martin
Oscar Ayo-Martin, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Francisco Hernández-Fernández
Francisco Hernández-Fernández, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jordi Blasco
Jordi Blasco, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Alejandro Rodríguez-Vázquez
Alejandro Rodríguez-Vázquez, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Antonio Cruz-Culebras
Antonio Cruz-Culebras, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Francisco Moniche
Francisco Moniche, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Joan Montaner
Joan Montaner, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Soledad Perez-Sanchez
Soledad Perez-Sanchez, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
María Jesús García Sánchez
María Jesús García Sánchez, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marta Guillán Rodríguez
Marta Guillán Rodríguez, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Katarina Jood
Katarina Jood, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Annika Nordanstig
Annika Nordanstig, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael V Mazya
Michael V Mazya, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Tiago TP Moreira
Tiago TP Moreira, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Gianmarco Bernava
Gianmarco Bernava, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Morin Beyeler
Morin Beyeler, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Manuel Bolognese
Manuel Bolognese, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Emmanuel Carrera
Emmanuel Carrera, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Tomas Dobrocky
Tomas Dobrocky, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Grzegorz Marek Karwacki
Grzegorz Marek Karwacki, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Emanuela Keller
Emanuela Keller, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Chang Yang Hsieh
Chang Yang Hsieh, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Surawan Boonyakarnkul
Surawan Boonyakarnkul, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anchalee Churojana
Anchalee Churojana, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ozlem Aykac
Ozlem Aykac, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Atilla Özcan Ozdemir
Atilla Özcan Ozdemir, M.D.
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Arsida Bajrami
Arsida Bajrami, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Songul Senadim
Songul Senadim, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Syed Irteza Hussain
Syed Irteza Hussain, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Seby John
Seby John, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Soma Banerjee
Soma Banerjee, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Joseph Kwan
Joseph Kwan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kailash Krishnan
Kailash Krishnan, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Robert Lenthall
Robert Lenthall, MBBS, MSc, FRCR, FRCP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ashok Matthews
Ashok Matthews, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ken Wong
Ken Wong, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Liqun Zhang
Liqun Zhang, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Dorothea Altschul
Dorothea Altschul, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kaiz S Asif
Kaiz S Asif, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Zeelalem Bahiru
Zeelalem Bahiru, NP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kristine Below
Kristine Below, BS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
José Biller
José Biller, MD, FACP, FAAN, FANA, FAHA
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sean Ruland
Sean Ruland, DO
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Saqib A Chaudry
Saqib A Chaudry, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael Chen
Michael Chen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Alex Chebl
Alex Chebl, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jackie Cibulka
Jackie Cibulka, RN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Leon Cistrunk
Leon Cistrunk
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Judith Clark
Judith Clark, RN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Marco Colasurdo
Marco Colasurdo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Alexandra Czap
Alexandra Czap, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adam de Havenon
Adam de Havenon, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Salvatore D'Amato
Salvatore D'Amato, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sushrut Dharmadhikari
Sushrut Dharmadhikari, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Kasey B Grimmett
Kasey B Grimmett, BSN, RN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adam A Dmytriw
Adam A Dmytriw, MD, MPH
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mark R Etherton
Mark R Etherton, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Chizoba Ezepue
Chizoba Ezepue, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mudassir Farooqui
Mudassir Farooqui, MD, MPH
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Steven K Feske
Steven K Feske, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Lauren Fink
Lauren Fink, BSN, RN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ulviyya Gasimova
Ulviyya Gasimova, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Amy K Guzik
Amy K Guzik, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Maryam Hakemi
Maryam Hakemi, BSN, MS, AGNP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Majesta Hovingh
Majesta Hovingh, MS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Muhib Khan
Muhib Khan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Dinesh Jillela
Dinesh Jillela, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Peter T Kan
Peter T Kan, MD, MPH
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rakesh Khatri
Rakesh Khatri, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ayaz M Khawaja
Ayaz M Khawaja, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Naim N Khoury
Naim N Khoury, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nicole L Kiley
Nicole L Kiley, PA-C
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Benny S Kim
Benny S Kim, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Murali K Kolikonda
Murali K Kolikonda, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Anna Luisa Kuhn
Anna Luisa Kuhn, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Stephanie Lara
Stephanie Lara, RN, MS-HCA, SCRN
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Guillermo Linares
Guillermo Linares, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Italo Linfante
Italo Linfante, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Timothy G Lukovits
Timothy G Lukovits, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sarah Lycan
Sarah Lycan, NP
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Shailesh S Male
Shailesh S Male, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Laith Maali
Laith Maali, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
John Mancin
John Mancin, DO
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hesham Masoud
Hesham Masoud, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ghada A Mohamed
Ghada A Mohamed, MD, MSc
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Andre Monteiro
Andre Monteiro, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Fadi Nahab
Fadi Nahab, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Krishna Nalleballe
Krishna Nalleballe, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Santiago Ortega-Gutierrez
Santiago Ortega-Gutierrez, MD MS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ajit S Puri
Ajit S Puri, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Yazan Radaideh
Yazan Radaideh, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Rahul H Rahangdale
Rahul H Rahangdale, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ansaar Rai
Ansaar Rai, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Pankajavalli Ramakrishnan
Pankajavalli Ramakrishnan, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aravind B Reddy
Aravind B Reddy, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Diana M Rojas-Soto
Diana M Rojas-Soto, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jose Rafael Romero
Jose Rafael Romero, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Natalia S Rost
Natalia S Rost, MD, MPH
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Aaron Rothstein
Aaron Rothstein, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Setareh Salehi Omran
Setareh Salehi Omran, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sunil A Sheth
Sunil A Sheth, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Adnan H Siddiqui
Adnan H Siddiqui, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Amy K Starosciak
Amy K Starosciak, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Nicholas E Tarlov
Nicholas E Tarlov, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Robert A Taylor
Robert A Taylor, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Michael J Wang
Michael J Wang, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Jared Wolfe
Jared Wolfe, BA
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Ka-Ho Wong
Ka-Ho Wong, MBA
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Huynh Vu Le
Huynh Vu Le, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Quy Viet Nguyen
Quy Viet Nguyen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Thong Nhu Pham
Thong Nhu Pham, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Trung Thanh Nguyen
Trung Thanh Nguyen, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Hoang Thi Phan
Hoang Thi Phan, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Mai Duy Ton
Mai Duy Ton, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Urs Fischer
Urs Fischer, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Patrik Michel
Patrik Michel, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Davide Strambo
Davide Strambo, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Sheila O Martins
Sheila O Martins, MD, PhD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Osama O Zaidat
Osama O Zaidat, MD, MS
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1,
Raul G Nogueira
Raul G Nogueira, MD
1From the Department of Neurology (T.N.N., P.K., Anvitha Sathya, H.C.L., Andreas Charidimou, Judith Clark, S.K.F., J.R.R.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiology (T.N.N., M.M.Q., P.K., Mohamad Abdalkader, Anvitha Sathya, H.C.L., N.L.K.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Radiation Oncology (M.M.Q.), Boston Medical Center, Boston University Chobanian & Avedisian School of Medicine; Department of Stroke Neurology (H.Y.), National Hospital Organization, Osaka National Hospital, Japan; Department of Neurology (R.M., Petra Sedova), International Clinical Research Centre, St Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic; 2nd Department of Neurology (Anna Czlonkowska, M.A.K.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Internal Medicine and Cardiology (Petra Sedova), University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic; Department of Neurology (O.Y.M.), Alexandria University Stroke and Neurointervention Unit, Egypt; Department of Neurology (H.R.V., K.U.), Cleveland Clinic; Centre Hospitalier de l'Université de Bordeaux (E.L.), INSERM, Bordeaux Population Health Research Center, France; Second Department of Neurology (Georgios Tsivgoulis, Lina Palaiodimou), “Attikon” University Hospital, National and Kapodistrian University of Athens, Greece; Ohio Health Riverside Methodist Hospital (A.I.L.); Neurology Department (J.D., Robin Lemmens), Leuven University Hospital, Belgium; University of Tennessee Health Science Center (V.I., N.G.); Cooper Neurological Institute (J.E.S., J.W.), Cooper University Hospital, Camden, NJ; Department of Neurology (Shadi Yaghi), Rhode Island Hospital, Brown University, Providence; Stroke Center (D.A.S.), Centro Hospitalar Universitário Lisboa Central—CHULC, Portugal, CEEM; Institute of Anatomy (D.A.S.), Faculdade de Medicina, Universidade de Lisboa, Portugal; Department of Neurology (Mahmoud Mohammaden, D.C.H.), Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA; Department of Neurology (E.S.K.), Akershus University Hospital, University of Oslo, Norway and Department of General Practice, University of Oslo, Norway; Division de Neurologia Vascular (V.P.L.), Departmento de Neurologia, Institute for Neurological Research—FLENI, Buenos Aires, Argentina; Stroke Unit (S.D.S.), Ramos Mejia Hospital, Buenos Aires, Argentina; Department of Medicine and Neurology (B.C.V.C.), Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia; Royal North Shore Hospital (Alice Ma), Sydney, Australia; Department of Neurophysiology (J.O.T), Liverpool Hospital, New South Wales, Australia; Department of Neurology (M.W.P.), Liverpool Hospital, New South Wales, Australia; Departments of Neurology (Shaloo Singal) and Radiology (L.-A.S.), Monash Medical Centre, Australia; Stroke and Aging Research Group (Shaloo Singhal), School of Clinical Sciences at Monash Health, Monash University, Australia; Department of Neurology (Rodrigo Tomazini Martins), Mater Hospital, Brisbane, Australia; Department of Neurology (Chris Enzinger), Medical University of Graz, Austria; Department of Neurology and Division of Neuroradiology (T.G.), Vascular and Interventional Radiology, Medical University of Graz, Austria; Department of Neurology (Aminur Rahman), Sir Salimullah Medical College, Mitford, Dhaka, Bangladesh; Hopital Erasme (T.B., N.L.), Brussels, Belgium; Department of Neurology (S.D.R., F.V.), Universitair Ziekenhuis Brussel, Center for Neurosciences, Vrije Universiteit Brussel, Belgium; Department of Neurology (P.V.), University Hospitals Antwerp; Department of Translational Neuroscience (P.V.), University of Antwerp, Belgium; Hospital das Clínicas (A.B.C., I.L.S.), São Paulo University, Brazil; Department of Neurology and Interventional Neuroradiology (R.C.T.H.), Hospital de Base de São José do Rio Preto, São Paulo, Brazil; Department of Neurology (L.O.N., I.B.S.), Hospital São Carlos, Fortaleza, Ceará, Brazil Stroke Unit (Rodrigo Targa Martins), Neurology, Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil; Moinhos de Vento Hospital (D.L.M.C.), Brazil; Hospital de Base do Distrito Federal (L.C.R.), Brasilia, Brazil; St. Anna University Hospital (T.S., Rosen Kalpachki, F.A.), Sofia, Bulgaria; Department of Neurology (Luciana Catanese), McMaster University, Hamilton, Canada; Department of Diagnostic Radiology (E.A.C.), Halifax Infirmary, Dalhousie University, Halifax, Canada; Departments of Clinical Neurosciences and Radiology (M.G., M.D.H.), Hotchkiss Brain Institute, Cummings School of Medicine, University of Calgary, Canada; Royal University Hospital (M.E.K., Lissa Peeling), Saskatoon, Saskatchewan, Canada; Department of Neurology (H.K.), Hurvitz Brain Sciences Program, Sunnybrook Health Sciences Centre, Division of Neurology, Department of Medicine, University of Toronto, Canada; Hopital Enfant Jesus (Pascale Lavoie), Centre Hospitalier de l'Université Laval, Quebec City, Canada; Toronto Western Hospital (A.P.), University of Toronto, Canada; Neuroradiology Department (R.R.), Instituto de Neurocirugía Dr. Asenjo, Santiago, Chile; Department of Neurology (H.-S.C.), General Hospital of Northern Theater Command, Shen Yang, China; Department of Neurology (Y.C., Shuiquan Yang), Foshan Sanshui District People's Hospital, China; Interventional Neuroradiology (X.H., Z.M.), Beijing Tiantian Hospital, China; Department of Neurology (M.R.B.), Sestre Milosrdnice University Hospital Center, Croatia; Department of Neurology (Marina Bralic), Clinical Hospital Center Rijeka, Croatia; Sveti Duh University Hospital - Zagreb (H.B.), Department of Neurology, Croatia; J.J. Strossmayer University of Osijek (H.B.), Faculty of Medicine, Department of Neurology and Neurosurgery, Osijek, Croatia; Department of Neurology (A.B.C.-Q.), Hospital Departamental Universitario del Quindío San Juan de Dios, Colombia; Department of Neurology (O.E.L.-S.), Clínica de la Costa, Barranquilla, Colombia; Department of Neurology (Martin Cabal), Faculty Hospital Ostrava, Czech Republic; Department of Neurology (D.T.), Blansko Hospital, Czech Republic; Oblastní Nemocnice Trutnov a.s. (Petr Fibrich), Czech Republic; Department of Neurology (R.H.), Comprehensive Stroke Center, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic; Neurology (H.H.), Hospital Příbram, Czech Republic; Uherskohradišťská Hospital (E.H.), Czech Republic; Department of Neurology (D.H.), Thomayer University Hospital Prague, Czech Republic; Neurocenter (L.J.), Regional Hospital Liberec, Czech Republic; Neurology (Jana Kadlcikova), Hospital Vyskov, Czech Republic; Regional Hospital Karlovy Vary (Igor Karpowicz), Czech Republic; Ostrava (L.K.), Czech Republic; Na Homolce Hospital (Martin Kovar), Czech Republic; Department of Neurology (D.L.), Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Czech Republic; Department of Neurology (J.N.E.S.O.), Krajská zdravotní, a.s.—Hospital Chomutov, Chomutov, Czech Republic; Karvina Mining Hospital (H.P.), Czech Republic; Neurology (Martin Reiser), České Budejovice Hospital, Czech Republic; Department of Neurology and Centre of Clinical Neuroscience (Petra Rekova), First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; Department of Neurology (V.R.), University Hospital Plzen, Czech Republic; Department of Neurology (O.S.), Hospital Jihlava, Czech Republic; Department of Neurology (M.Š.), Masaryk University Faculty of Medicine, University Hospital Brno, Czech Republic; Oblastni Nemocnice Kladno (L.S.), Czech Republic; Central Military Hospital in Prague (Martin Sramek), Czech Republic; Nemocnice Třinec (Lenka Zakova), Czech Republic; Copenhagen University Hospital (H.C., N.D.), Denmark; Stroke Center (H.K.I., T.C.T.), Rigshospitalet, University of Copenhagen, Denmark; Neurovascular Center (T.W.), Zealand University Hospital, University of Copenhagen, Roskilde, Denmark; El hussein Alzahar University Hospital (Khalid Sobh), Egypt; Neurocenter (P.Y.), Turku University Hospital, Turku University, Finland; Department of Radiology (K.A.), Turku University Hospital, Finland; Department of Neurology (Daniel Strbian), Helsinki University Hospital and University of Helsinki, Finland; Bayonne Hospital Neurology (P.B.), Stroke Unit, France; Libourne Hospital Neurology (Philippe Casenave), Stroke Unit, France; Centre Hospitalier d’Arcachon—Neurologie (Maria Dan), France; Agen Hospital Neurology (J.-M.F.), Stroke Unit, France; Neuroradiologie Interventionelle (J.-C.G.), Brest University Hospital, France; Neurochirurgie (E.M.), Brest University Hospital, France; Department of Neurology (C.S.), Rothschild Foundation Hospital, Paris, France; Lariboisiere Hospital (Peggy Reiner), Paris, France; Bordeaux University Hospital Neurology (F.R.), Stroke Unit, France; Department of Neurology (F.O.B.), University Hospital, Goethe University, Frankfurt, Germany; Department of Neurology (Stefan Boskamp, Michael Rosenkranz), Albertinen Krankenhaus, Hamburg, Germany; Department of Neurology and Stroke (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Hertie Institute for Clinical Brain Research (Joshua Mbroh, S.P.), Eberhard-Karls University, Tuebingen, Germany; Department of Neurology (Simon Nagel), Klinikum Ludwigshafen, Ludwigshafen/Rhein, Germany; Department of Neurology (Simon Nagel, P.A.R.), Heidelberg University Hospital, Germany; Department of Neurology and Experimental Neurology (C.H.N.), Center for Stroke Research Berlin, Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf (Götz Thomalla), Klinik und Poliklinik für Neurologie, Hamburg, Germany; Second Department of Neurology (Theodoros Karapanayiotides, Ioanna Koutroulou), AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Faculty of Health Sciences, Greece; Stroke Unit (O.K.), Metropolitan Hospital, Piraeus, Greece; Hospital General San Juan de Dios (J.D.B.G.), Guatemala; Mazumdar Shaw Medical Center (V.H.), Bangalore, Karnataka, India; Department of Neurology (Bindu Menon), Apollo Specialty Hospitals Nellore, Andhra Pradesh, India; Department of Neurology (Shashank Nagendra), Grant Medical College and Sir JJ Hospital, Mumbai, India; Mazumdar Shaw Medical Center (C.P.), Bangalore, Karnataka, India; Sree Chitra Tirunal Institute for Medical Sciences and Technology (P.N.S.), Trivandrum, Kerala, India; Department of Neurology (N.A.K.P.), Faculty of Medicine Udayana University, Sanglah General Hospital Denpasar, Bali, Indonesia; Dr. Soetomo General Hospital Surabaya (A.F.S.), Universitas Airlangga, Indonesia; Stroke Research Group (A.G.), Head of Stroke Care Unit, Department of Neurology, Vali-e-Asr Hospital, School of Medicine, Zanjan University of Medical Sciences, Iran; Neurosciences Research Center (Mehdi Farhoudi, E.S.H.), Imam Reza Hospital, Tabriz University of Medical Sciences, Iran; Neurology Department (T.A.R., S.I.S.), Hillel Yaffe Medical Center, Hadera, Israel; Neurointerventional Unit (S.A.K., Levite Ronen), Shamir Medical Center, Israel; Stroke Unit (Maurizio Acampa), University of Siena, Italy; Stroke Center IRCCS Sacro Cuore Don Calabria (A.A.), Verona, Italy; Dipartimento di Neuroradiologia Diagnostica e Interventistica Policinico Universitario IRCCS San Martino (Lucio Castellan, C.R.B.), Genova, Italy; Neurology and Stroke Unit (M.L., Michele Romoli), Ospedale Bufalini, Cesena, Italy; Neuroscience Section (R.O., Simona Sacco), Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, Italy; Interventional Neurovascular Unit (Leonardo Renieri), Careggi University Hospital, Florence, Italy; Neurological Department (Andrea Salmaggi, Davide Sangalli), “Alessandro Manzoni” Hospital, ASST Lecco, Via dell'Eremo, Italy; IRCCS Istituto delle Scienze Neurologiche di Bologna (A.Z.), Neurologia e Rete Stroke Metropolitana, Italy; Department of Neurosurgery (R.D., N.K.), Iwate Prefectural Central Hospital, Japan; Department of Neurology (H.F.), Japanese Red Cross Matsue Hospital, Japan; Department of Neurosurgery (T.F.), National Hospital Organization Osaka National Hospital, Japan; Department of Endovascular Surgery (K.F.), Tokyo Medical and Dental University, Japan; Department of Neurosurgery (H.I., N.S.), Kobe City Medical Center General Hospital, Japan; NTT Medical Center Tokyo (Takuya Kanamaru), Japan; Department of Neurology (Ryuhei Kono), Kin-ikyo Chuo Hospital, Japan; Shiroyama Hospital (K.M.), Japan; Department of Neurology (Manabu Sakaguchi), Osaka General Medical Center, Japan; Department of Neurology (Kenichiro Sakai), The Jikei University School of Medicine, Japan; Department of Neurology (Kazutaka Sonoda), Saiseikai Fukuoka General Hospital, SFGH, Japan; Stroke Center (Kenichi Todo), Osaka University Hospital, Japan; Department of Neurology (Fumio Miyashita), Kagoshima City Hospital, Japan; Department of Neurology (N.T.), Japanese Red Cross Kyoto Daini Hospital, Japan; Department of Stroke and Cerebrovascular Diseases (Y.M.), University of Tsukuba Hospital, Japan; Comprehensive Strokology (S.M.), Fujita Health University School of Medicine, Toyoake, Japan; Department of Neurology (N.O.), Kobe City Medical Center General Hospital, Japan; Department of Neurology (Seigo Shindo), Japanese Red Cross Kummoto Hospital, Japan; Osaka Red Cross Hospital (Y.T.), Japan; Department of Neurology (Takeshi Yoshimoto), National Cerebral and Cardiovascular Center, Japan; Department of Cerebrovascular Medicine (Kazunori Toyoda), National Cerebral and Cardiovascular Center, Japan; Cerebrovascular Medicine (T.U.), Stroke Center, Saga Medical Centre Koseikan, Japan; Stroke Medicine (Yoshiki Yagita), Kawasaki Medical School, Japan; Department of Neurology and Stroke Treatment (Takehiro Yamada), Japanese Red Cross Kyoto Daiichi Hospital, Japan; Advanced Brain Research (N.Y.), Tokushima University, Japan; Yokohama Brain and Spine Center (R.Y.), Japan; Department of Stroke Neurology (Yukako Yazawa), Kohnan Hospital, Sendai, Japan; Toyonaka Municipal Hospital (Y.S.), Japan; Kenyatta University Teaching (P.K.W.), Referral and Research Hospital, Kenya; Department of Neurology (J.-H.B.), Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea; Department of Neurology (S.B.L.), Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, South Korea; Department of Neurology (K.-D.S.), National Health Insurance Service Ilsan Hospital, Goyang, Korea; Department of Neurology (S.-I.S.), Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea; Department of Urgent Neurology (A.A.A.), University Clinic of Neurology, University Ss. Cyril and Methodius-Faculty of Medicine, Skopje, North Macedonia; Hospital Sultan Abdul Halim (Y.C.C.), Malaysia; Department of Medicine (W.A.W.Z.), National University of Malaysia; Department of Surgery (A.S.J.), National University of Malaysia; Neurovascular Unit and Neurology Department (F.G.-R., A.I.-V.), University Hospital Jose Eleuterio Gonzalez, Universidad Autonoma de Nuevo Leon, Mexico; Department of Neurology (M.M.-M.), Hospital de especialidades del Centro Médico Nacional Siglo XXI IMSS, Mexico; “Nicolae Testemitanu” State University of Medicine and Pharmacy (S.G., Pavel Leahu); Emergency Medicine Institute (L.P., S.G.), Chisinau, Republic of Moldova; Amsterdam University Medical Centers Amsterdam (J.M.C., L.A.R.), the Netherlands; Departments of Neurology (D.W.J.D.) and Neurology and Radiology (D.H.K.D.-N.), Erasmus University Medical Center Rotterdam, the Netherlands; Department of Medicine (Annemarei Ranta), University of Otago—Wellington, and Department of Neurology, Wellington Hospital, New Zealand; Department of Neurology (T.Y.W.), Christchurch Hospital, New Zealand; Department of Medical Records (T.T.A.), Federal Medical Centre, Owo, Ondo State, Nigeria; Neurology Unit (A.H.B., K.W.W.), University of Ilorin, Nigeria; Neurology Unit (E.O.N.), Department of Medicine, Federal Medical Centre Owerri, Neurology Unit, Federal Medical Centre, Owerri, Nigeria; Neurology Unit, Department of Medicine, (T.A.S.) Federal Medical Center, Owo, Ondo State, Nigeria; Department of Neurology (O.M.R.), Akershus University Hospital, Norway, and Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology (E.C.S.), Oslo, Oslo, Norway and The Norwegian Air Ambulance Foundation, Norway; Central Stroke Unit (A.M.A.H.), Neuroscience Directorate, Khoula Hospital, Ministry of Health, Oman; Lahore General Hospital (S.A., U.R.), Pakistan; Departamento de Neurología (L.R.-K., M.Á.V.), Hospital Nacional Edgardo Rebagliati Martins,Essalud, Lima, Perú; St. Luke's Medical Center (P.M.Y.), Global City, Philippines; St. Luke's Medical Center (J.S.H.D., M.C.P.-F., C.O.C.), Quezon City, Philippines; Department of Neurology (W.B.), Specialist Hospital Konskie, Collegium Medicum, Jan Kochanowski University, Kielce, Poland; Clinic of Neurology (Aleksander Debiec, J.S.), Military Institute of Medicine, Warsaw, Poland; Department of Neurology (Malgorzata Dorobek), Central Clinical Hospital of the Ministry of Interior and Administration (M.D.), Warsaw, Poland; Department of Neurology (B.M.L.-R.), St. Jadwiga Provincial Specialist Hospital in Opole, Institute of Medical Sciences, University of Opole, Poland; Department of Neurology (A.L.-B.), Leszek Giec Upper Silesian Medical Centre of the Silesian Medical University in Katowice, School of Health Sciences, Medical University of Silesia in Katowice, Poland; 1st Department of Neurology (H.S.-J.), Institute of Psychiatry and Neurology, Warsaw, Poland; Department of Neurology in Sandomierz (Piotr Sobolewski), Collegium Medicum, Jan Kochanowski University in Kielce, Sandomierz, Poland; Department of Neurology (M.W.), Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszow, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland; Department of Neurology (J.Z.-T.), Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland; Neuroradiology Department—Centro Hospitalar de Vila Nova de Gaia/Espinho (A.P.A.), Portugal; Neurology Department (Mariana Rocha), Centro Hospitalar Vila Nova de Gaia/Espinho; Department of Neurology (Pedro Castro), Centro Hospitalar Universitário de São João, Portugal; Stroke Unit—Hospital Pedro Hispano (V.T.C., P.V.F.), Matosinhos, Portugal; Stroke Unit (Patricia Ferreira, A.P.N.), Centro Hospitalar Universitário de Lisboa Central, Portugal; Stroke Unit (Luisa Fonseca), Medicine Department, Centro Hospitalar Universitário de São João, Portugal; Department of Neurology (J.P.M.), Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, Lisbon, Portugal; Hospital de Santa Maria—Centro Hospitalar Lisboa Norte (T.P.M.), Portugal; Department of Neurology (Miguel Rodrigues), Hospital Garcia de Orta, Almada, Portugal; Centro Hospitalar Universitário de São João (M.L.S.), Portugal; Bucarest University Emergency Hospital (Adela Dimitriade), Romania; Department of Neurology (C.F.-P.), County Clinic Hospital, Faculty of Medicine, Transilvania University, Brasov, Romania; Department of Neurology King Fahad Hospital of the University Imam Abdulrahman bin Faisal University Dammam (M.A.H.), Saudi Arabia; Raffles Hospital (N.V.), Singapore; Department of Neurology (G.K., Miroslav Mako), Faculty Hospital Trnava, Slovakia; Jessenius Medical Faculty (G.K., M.M.), Martin, Commenius University, Bratislava, Slovakia; Department of Neurology (G.K.), Slovak Medical University, Bratislava; Complejo Hospitalario Universitario de Albacete (O.A.-M., F.H.-F.), Albacete, Spain; Interventional Neuroradiology (J.B.), Hospital Clínic de Barcelona, Spain; Comprehensive Stroke Center (A.R.-V.), Hospital Clínic de Barcelona, Spain; Department of Neurology (Unidad de Ictus) (A.C.-C.), Hospital Universitario Ramón y Cajal, Madrid, Spain; Stroke Unit (Francisco Moniche), Neurology Department, Hospital Universitario Virgen del Rocío, Spain; Department of Neurology (Joan Montaner, S.P.-S.), Hospital Universitario Virgen Macarena & Neurovascular Research Laboratory, Instituto de Biomedicina de Sevilla-IbiS, Spain; Department of Neuroradiology (M.J.G.S.), Hospital Universitario Rey Juan Carlos, Spain; Department of Neurology (M.G.R.), Hospital Universitario Rey Juan Carlos, Spain; Institute of Neuroscience and Physiology (K.J., A.N.), Department of Clinical Neuroscience, Sahlgrenska Academy, University of Gothenburg; Department of Neurology (K.J., A.N.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden; Department of Neurology (M.V.M., T.T.P.M.), Karolinska University Hospital & Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Interventional Neuroradiology (G.B.), University Hospitals of Geneva, Switzerland; Department of Neurology (Morin Beyeler, U.F.), Inselspital, Bern University Hospital; Neurocenter (Manuel Bolognese), Cantonal Hospital of Lucerne, Switzerland; Department of Neurology (E.C.), University Hospitals of Geneva, Switzerland; Diagnostic and Interventional Neuroradiology (T.D.), Bern University Hospital, University of Bern, Switzerland; Radiology and Nuclear Medicine (G.M.K.), Cantonal Hospital of Lucerne, Switzerland; University Hospital (E.K.), University of Zurich, Switzerland; Department of Neurology (C.Y.H.), Tainan Sin Lau Hospital, Taiwan; Department of Radiology (Surawan Boonyakarnkul), Ramathibodi Hospital, Mahidol University, Thailand; Department of Radiology (Anchalee Churojana), Siriraj Hospital, Mahidol University, Thailand; Eskisehir Osmangazi University (O.A., M.D.O.), Turkey; Istanbul Aydın University (A.B., Songul Senadim), Florya Medicalpark Stroke Center, Turkey; Cleveland Clinic Abu Dhabi (S.I.H., S.J.), UAE; Department of Stroke Medicine (Soma Banerjee, Joseph Kwan), Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, United Kingdom; Stroke (K.K.), Department of Medicine (K.K.), Nottingham University Hospitals NHS Trust, United Kingdom; Neuroradiology (Robert Lenthall), Nottingham University Hospitals NHS Trust, United Kingdom; Barking Havering and Redbridge University Hospitals (Ashok Matthews), Romford, United Kingdom; Royal London Hospital (K.W.), Barts Health NHS Trust, United Kingdom; St George's University Hospital (Liqun Zhang), London, United Kingdom; Valley Hospital Health System (D.A.), Neurosurgeons of NJ, New Jersey; Amita Health and University of Illinois-Chicago (K.S.A.); Inova Fairfax Hospital (Z.B.), Virginia; Neuroscience and Stroke Program (K.B., O.O.Z.), Bon Secours Mercy Health St Vincent Hospital, Toledo, OH; Loyola University Chicago Stritch School of Medicine (J.B.A.N.A., S.R.), Illinois; Inova Fairfax Hospital (S.A.C.), University of Virginia School of Medicine, Virginia; Rush University Medical Center (Michael Chen, Y.R.), Chicago, IL; Department of Neurology (Alex Chebl), Henry Ford Health System, Detroit, MI; Clinical Data Specialist (Jackie Cibulka), EBC Data Abstraction, University of Tennessee Health Science Center; Solution Architect II (Leon Cistrunk), Clinical Decision Support, University of Tennessee Health Science Center; Department of Radiology (Marco Colasurdo), University of Texas Medical Branch, Galveston, TX; Neurology (Alexandra Czap, S.A.S.), UTHealth McGovern Medical School, Houston, TX; Department of Neurology (A.H.), Yale University School of Medicine, New Haven; Brigham and Women's Hospital (S.D.A., A.A.D.), Boston; Baptist Health Medical Center—Little Rock (S.D., K.B.G.), Little Rock, AR; Neurointerventional Program (A.A.D.), Departments of Medical Imaging and Clinical Neurological Sciences, London Health Sciences Centre, Western University, London, ON; Neuroendovascular Program (A.A.D.), Massachusetts General Hospital & Brigham and Women's Hospital, Harvard Medical School, Boston; Department of Neurology (M.R.E., N.S.R.), Massachusetts General Hospital, Boston; Department of Interventional and Vascular Neurology (Chizoba Ezepue), Neuroscience Center, SSM Health DePaul Hospital, St. Louis, Missouri; University of Iowa (Mudassir Farooqui, S.O.G.); Santa Barbara Cottage Hospital (Lauren Fink, R.A.T.), California; Department of Neurology (U.G.), Saint Louis University School of Medicine, Missouri; Department of Neurology (A.K.G., S.L.), Wake Forest Baptist Medical Center, NC; Wayne State University (M.H.G.N.P., A.M.K.), Detroit, MI; Department of Neurosciences and Comprehensive Stroke Center (M.H., Muhib Khan), Spectrum Health and Michigan State University College of Human Medicine; Department of Neurology (D.J.), Grady Memorial Hospital, Emory University School of Medicine; Department of Neurosurgery (P.T.K.), University of Texas Medical Branch; Department of Neurology (Rakesh Khatri), Texas Tech University Health Science Center, El Paso; HSHS St. John's Hospital (N.N.K.), Southern Illinois University School of Medicine, Springfield; Virginia Hospital Center (B.S.K.); Baptist Health Medical Group (M.K.K.), Baptist Health Lexington, KY; Division of Neurointerventional Radiology (A.L.K., A.S.P.), Department of Radiology, University of Massachusetts Medical Center, Worcester, MA; Community Memorial Hospital (S.L.-H.C.A.,S.C.R.N., N.E.T.), Ventura, CA; Vascular and Neurointerventional Services (G.L.), Saint Louis University, Missouri; Miami Cardiac & Vascular Institute (I.L.), Miami Neuroscience Institute, FL; Dartmouth Hitchcock Medical Center (T.G.L., D.M.R.-S.), Lebanon, NH; Department of Neurology & Neurosurgery (S.S.M.), ECU Health Medical Center, Greenville, NC; Department of Neurology (L.M.), University of Kansas Medical Center; West Virginia University (John Mancin, Ansaar Rai); Department of Neurology (H.M., A.B.R.), SUNY Upstate, Syracuse, NY; Department of Neurology (G.A.M.), Medical University of South Carolina; Department of Neurosurgery (Andre Monteiro, A.H.S.), University at Buffalo; Department of Neurology (F.N.), Pediatrics, Emory University, Georgia; Department of Neurology (K.N.), University of Arkansas for Medical Sciences, Arkansas; Ascension St Johns Medical Center (R.H.R.), Tulsa, OK; Riverside Regional Medical Center (Pankajavalli Ramakrishnan), Newport News, VA; Department of Neurology (Aaron Rothstein), Hospital of the University of Pennsylvania, Philadelphia; University of Colorado School of Medicine (S.S.O.), Aurora, CO; Miami Neuroscience Institute (A.K.S.), Miami; UNC School of Medicine (M.J.W.), North Carolina; University of Utah (K.-H.W.); Hue Central (H.V.L., Q.V.N.), Vietnam; Da Nang Hospital (T.N.P., T.T.N.), Da Nang, Vietnam; Bach Mai Hospital (H.T.P., M.D.T.), Hanoi, Vietnam; Department of Neurology (U.F.), Basel University Hospital, University of Basel, Switzerland; Neurology Service (P.M., Davide Strambo), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne, Switzerland; Department of Neurology (S.O.M.), Federal University of Rio Grande do Sul, Porto Alegre; Hospital de Clínicas de Porto Alegre (S.O.M.), Brazil; and Department of Neurology (R.G.N.), University of Pittsburgh Medical Center, Pittsburgh. Ondrej Skoda is currently at the Department of Neurology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
1;
and the SVIN COVID-19 Global Stroke Registry1